For more than a century, biologists have wondered what the earliest animals were like when they first arose in the ancient oceans over half a billion years ago.
Searching among today’s most primitive-looking animals for the earliest branch of the animal tree of life, scientists gradually narrowed the possibilities down to two groups: sponges, which spend their entire adult lives in one spot, filtering food from seawater; and comb jellies, voracious predators that oar their way through the world’s oceans in search of food.
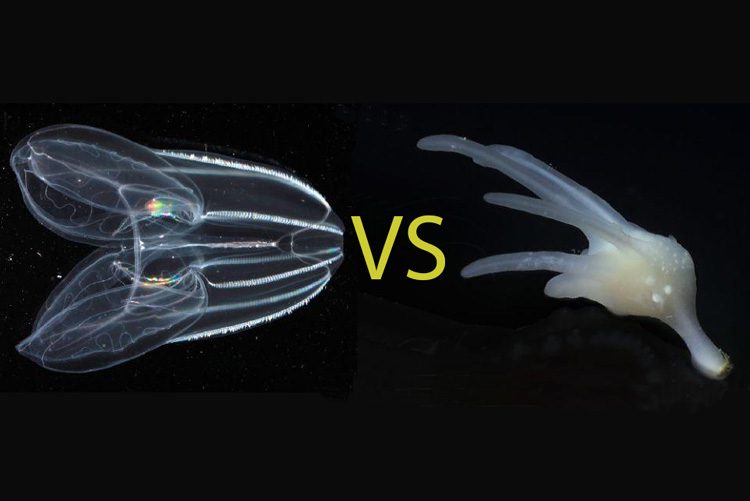
In a new study published this week in the journal Nature, researchers use a novel approach based on chromosome structure to come up with a definitive answer: Comb jellies, or ctenophores (teen’-a-fores), were the first lineage to branch off from the animal tree. Sponges were next, followed by the diversification of all other animals, including the lineage leading to humans.
Although the researchers determined that the ctenophore lineage branched off before sponges, both groups of animals have continued to evolve from their common ancestor. Nevertheless, evolutionary biologists believe that these groups still share characteristics with the earliest animals, and that studying these early branches of the animal tree of life can shed light on how animals arose and evolved to the diversity of species we see around us today.

“The most recent common ancestor of all animals probably lived 600 or 700 million years ago. It’s hard to know what they were like because they were soft-bodied animals and didn’t leave a direct fossil record. But we can use comparisons across living animals to learn about our common ancestors,” said Daniel Rokhsar, University of California, Berkeley professor of molecular and cell biology and co-corresponding author of the paper along with Darrin Schultz and Oleg Simakov of the University of Vienna. “It’s exciting — we’re looking back deep in time where we have no hope of getting fossils, but by comparing genomes, we’re learning things about these very early ancestors.”
Understanding the relationships among animal lineages will help scientists understand how key features of animal biology, such as the nervous system, muscles and digestive tract, evolved over time, the researchers say.
“We developed a new way to take one of the deepest glimpses possible into the origins of animal life,” said Schultz, the lead author and a former UC Santa Cruz graduate student and researcher at the Monterey Bay Aquarium Research Institute (MBARI) who is now a postdoctoral researcher at the University of Vienna. “This finding will lay the foundation for the scientific community to begin to develop a better understanding of how animals have evolved.”
What’s an animal?
Most familiar animals, including worms, flies, mollusks, sea stars and vertebrates — and including humans — have a head with a centralized brain, a gut running from mouth to anus, muscles and other shared features that had already evolved by the time of the famed “Cambrian Explosion” around 500 million years ago. Together, these animals are called bilaterians.
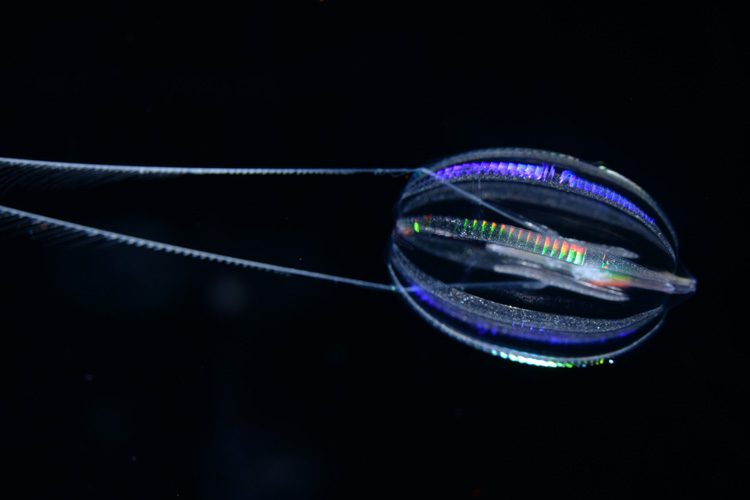
Other bona fide animals, however, such as jellyfish, sea anemones, sponges and ctenophores, have simpler body plans. These creatures lack many bilaterian features — for example, they lack a defined brain and may not even have a nervous system or muscles — but still share the hallmarks of animal life, notably the development of multicellular bodies from a fertilized egg.
The evolutionary relationships among these diverse creatures — specifically, the order in which each of the lineages branched off from the main trunk of the animal tree of life — has been controversial.
With the rise of DNA sequencing, biologists were able to compare the sequences of genes shared by animals to construct a family tree that illustrates how animals and their genes evolved over time since the earliest animals arose in the Precambrian Period.
But these phylogenetic methods based on gene sequences failed to resolve the controversy over whether sponges or comb jellies were the earliest branch of the animal tree, in part because of the deep antiquity of their divergence, Rokhsar said.
“The results of sophisticated sequence-based studies were basically split,” he said. “Some researchers did well-designed analyses and found that sponges branched first. Others did equally complex and justifiable studies and got ctenophores. There hasn’t really been any convergence to a definitive answer.”

Just looking at them, sponges seem quite primitive. After their free-swimming larval stage, they settle down and generally remain in one place, gently sweeping water through their pores to capture small food particles dissolved in sea water. They have no nerves or muscles, though their hard parts make nice scrubbers in the bath.
“Traditionally, sponges have been widely considered to be the earliest surviving branch of the animal tree, because sponges don’t have a nervous system, they don’t have muscles, and they look a little bit like colonial versions of some unicellular protozoans,” Rokhsar said. “And so, it was a nice story: First came the unicellular protozoans, and then sponge-like multicellular consortia of such cells evolved and became the ancestor of all of today’s animal diversity. In this scenario, the sponge lineage preserves many features of the animal ancestor on the branch leading to all other animals, including us. Specializations evolved that led to neurons, nerves and muscles and guts and all those things that we know and love as the defining features of the rest of animal life. Sponges appear to be primitive, since they lack those features.”
The other candidate for earliest animal lineage is the group of comb jellies, popular animals in many aquariums. While they look superficially like jellyfish — they often have a bell-like shape, although with two lobes, unlike jellyfish, and usually tentacles — they are only distantly related. And while jellyfish squirt their way through the water, ctenophores propel themselves with eight rows of beating cilia arranged down their sides like combs. Along the California coast, a common ctenophore is the 1-inch-diameter sea gooseberry.
Chromosomes to the rescue
To learn whether sponges or ctenophores were the earliest branch of animals, the new study relied on an unlikely feature: the organization of genes into chromosomes. Each species has a characteristic chromosome number — humans have 23 pairs — and a characteristic distribution of genes along chromosomes.
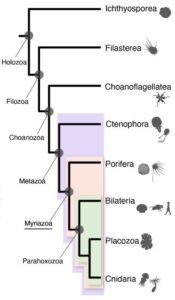
Rokhsar, Simakov and collaborators had previously shown that the chromosomes of sponges, jellyfish and many other invertebrates carry similar sets of genes, despite more than half a billion years of independent evolution. This discovery suggested that chromosomes of many animals evolve slowly, and allowed the team to computationally reconstruct the chromosomes of the common ancestor of these diverse animals.
But the chromosome structure of ctenophores was unknown until 2021, when Schultz — then a graduate student at UC Santa Cruz — and his co-advisers, Richard Green of UCSC and Steven Haddock of MBARI and UCSC, determined the chromosome structure of the ctenophore Hormiphora californensis. It looked very different from those of other animals, which posed a puzzle, Rokhsar said.
“At first, we couldn’t tell if ctenophore chromosomes were different from those of other animals simply because they’d just changed a lot over hundreds of millions of years,” Rokhsar explained. “Alternatively, they could be different because they branched off first, before all other animal lineages appeared. We needed to figure it out.”
The researchers joined forces to sequence the genomes of another comb jelly and sponge, as well as three single-celled creatures that are outside the animal lineage: a choanoflagellate, a filasterean amoeba and a fish parasite called an ichthyosporean. Rough genome sequences of these non-animals already existed, but they did not contain the critical information needed for chromosome-scale gene linkage: where they sit on the chromosome.
A smoking gun
Remarkably, when the team compared the chromosomes of these diverse animals and non-animals, they found that ctenophores and non-animals shared particular gene-chromosome combinations, while the chromosomes of sponges and other animals were rearranged in a distinctly different manner.

“That was the smoking gun — we found a handful of rearrangements shared by sponges and non-ctenophore animals. In contrast, ctenophores resembled the non-animals. The simplest explanation is that ctenophores branched off before the rearrangements occurred,” he said.
“The fingerprints of this ancient evolutionary event are still present in the genomes of animals hundreds of millions of years later,” Schultz said. “This research … gives us context for understanding what makes animals animals. This work will help us understand the basic functions we all share, like how they sense their surroundings, how they eat and how they move.”
Rokhsar emphasized that the team’s conclusions are robustly based on five sets of gene-chromosome combinations.
“We found a relic of a very ancient chromosomal signal,” he said. “It took some statistical detective work to convince ourselves that this really is a clear signal and not just random noise, because we’re dealing with relatively small groups of genes and perhaps a billion years of divergence between the animals and non-animals. But the signal is there and strongly supports the ‘ctenophore-branched-first’ scenario. The only way the alternative sponge-first hypothesis could be true would be if multiple convergent rearrangements happened in both sponges and non-ctenophore animals, which is very unlikely.”
Jessen Bredeson of UC Berkeley also contributed to this work.
Funding for this research was provided by the David and Lucile Packard Foundation, MBARI, the National Science Foundation (GRFP DGE 1339067 and DEB-1542679), the European Research Council’s Horizon 2020: European Union Research and Innovation Programme (grant No. 945026), internal funds of the Okinawa Institute of Science and Technology Molecular Genetics Unit, the Chan Zuckerberg Biohub Network and the Marthella Foskett Brown Chair in Biological Sciences.