LIC Tags
| LicV1 Forward | TACTTCCAATCCAATGCA |
| LicV1 Reverse | TTATCCACTTCCAATGTTATTA |
| GFP Reverse | CTCCCACTACCAATGCC |
| LicV2 Forward | TTTAAGAAGGAGATATAGATC |
| LicV2 Reverse T | TTATGGAGTTGGGATCTTATTA |
These are just a few examples of LIC tags that we use. For more information about other LIC versions, see our Expression Vector Lists here.
PCR Reaction
| Volume (ul) | Reagent |
|---|---|
| 2.0 | 1ng/ul plasmid template |
| 5.0 | PfuUltraII 10x Buffer |
| 0.4 | dNTPs (100mM) |
| 2.0 | Primer 1 (at 5uM) |
| 2.0 | Primer 2 (at 5uM) |
| 1.0 | PfuUltra II enzyme |
| 37.6 | H2O |
| 50 total |
Cycle At:
| 95C | 3min | ||
| 95C | 30sec | ||
| 55C | 30sec | 30 cycles | |
| 72C | 15sec/kb | ||
| 72C | 3min |
PCR Purify
Run the PCR on a gel to verify that the reaction worked. Use a Machery-Nagel pcr purification kit (or similar) to do a PCR cleanup reaction. It is absolutely essential that NO free dNTPs are in your PCR product.
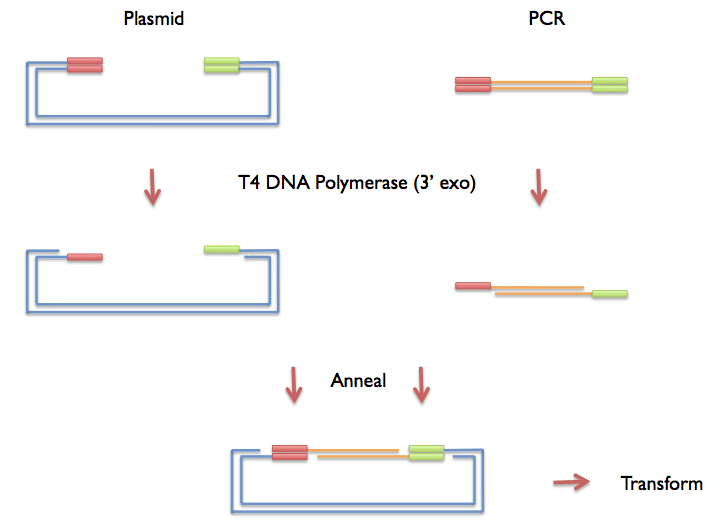
LIC Reaction for Vector
| Volume (ul) | Reagent |
|---|---|
| 10 | Linearized vector |
| 2 | T4 buffer |
| 2 | dGTP** (25mM) |
| 1 | 100mM DTT |
| 0.2 | T4 Polymerase enzyme |
| 4.8 | H20 |
| 20 total |
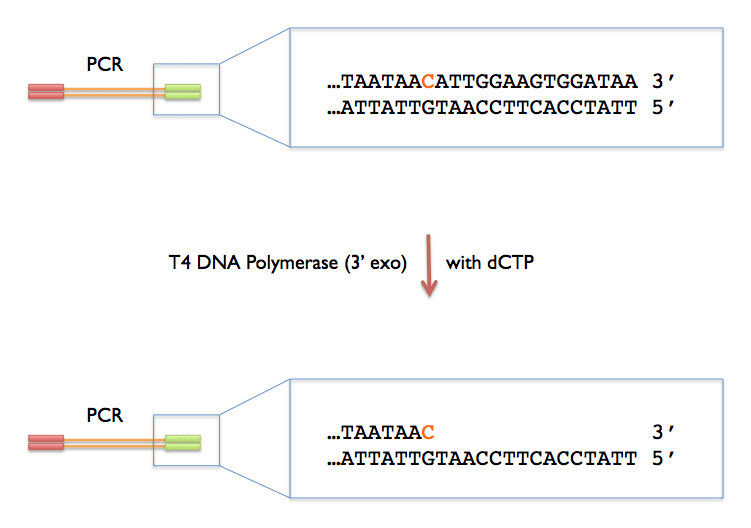
LIC Reaction for Insert
| Volume (ul) | Reagent |
|---|---|
| 5 | Purified PCR |
| 2 | T4 buffer |
| 2 | dCTP** (25mM) |
| 1 | 100mM DTT |
| 0.2 | T4 Polymerase enzyme |
| 9.8 | H20 |
| 20 total |
Incubate at 22C for 40min, then heat-denature the enzyme at 75C for 20min**NOTE: If you are using vector 2A-T (untagged), you must switch nucleotides (i.e., add dCTP to the vector and dGTP to the insert).
Important: T4 polymerase must be “LIC-qualified”. You can get this product from EMD Biosciences / Novagen here.
Anneal & Transform
Combine 3ul LICed insert + 3ul LICed vector in 20ul total volume, incubate 5-30min at room temperature to anneal.
Transform 6ul into XL-1 Blues or similar cloning strain:
1. Add 6ul to 100ul of competent cells on ice
2. Incubate on ice for 30min
3. Heat shock at 42C for 30sec
4. Immediately place on ice for 2min
5. Add 900ul 2YT and shake for 1hr at 37C
6. Plate 150ul on appropriate antibiotic plate
You can download the LIC Cloning Protocol here.
For more detailed information about LIC and SLIC (Sequence and Ligation Independent Cloning), please refer to our manual that you can download here.