Analysis Techniques
Please feel free to ask one of the facility staff if you are not sure which ionization method would be best for your sample. In general, if your sample decomposes while obtaining a melting point, then you will not be able to request electron impact (EI). Instead electrospray ionization (ESI) will be required. All salts require ESI.
The electron impact (EI) technique is fairly straightforward. The sample must be delivered as a gas, which is usually accomplished by heating the sample to vaporize it from the probe. Once in the gas phase, the compound passes into an electron ionization region where it interacts with a beam of electrons of nearly homogeneous energy (70 electron volts), typically causing electron ejection and some degree of fragmentation. EI is most useful for compounds below a molecular weight of 400 Da because larger molecules tend to thermally decompose during vaporization. EI is principally used as a detector for gas chromatography (GC/MS) in a wide variety of areas including synthetic organic chemistry, hydrocarbons analysis, pharmaceutical compounds and drugs of abuse (for example, it is widely used in the Olympic drug testing program), and environmental studies such as water testing. Non-air sensitive samples for EI analysis can be submitted in an Eppendorf tube (available in the Facility along with the submission forms) or any other small vial. The staff will transfer the appropriate amount to special capillaries prior to analysis. Sample requirements for EI analysis are on the order of 50 micrograms (µg) up to a maximum of 1 mg.
Our magnetic sector mass spectrometer is equipped with a chemical ionization source. CI is applied to samples similar to those analyzed by EI and is primarily used to enhance the abundance of the molecular ion. As with EI, CI requires that the samples are volatile. Samples need to be thermally stable since vaporization within the CI source occurs during heating. CI uses gas phase ion-molecule reactions within the vacuum of the mass spectrometer to produce ions from the sample molecules. The chemical ionization process is initiated with a reagent gas such as methane, isobutane, or ammonia, which is ionized by electron impact. The high gas pressure in the ion source results in ion-molecule reactions between the reagent gas ions and the reagent gas molecules. Some of the products of the ion-molecule reactions can react with the sample molecules to produce ions.
The Mass Spectrometry Facility primarily uses methane as the reagent gas. A possible mechanism for the CI process is:
CH4 + e- → CH4+ + 2 e-
CH4+ + CH4 → CH5+ + CH3
CH5+ + M → CH4 + MH+ (protonation of the sample molecule, M)
In contrast with EI, the molecular species are all even – electron ions. There is no electron transfer involved in the formation of the molecular species. This means that CI molecular species tend to be relatively stable when compared with their analogous molecular ion. This improves the likelihood of detecting the molecular species and so compared with EI, molecular peaks tend to be intense and fragmentation relatively sparse. When fragmentation is observed, it is frequently due to the loss of small neutral molecules (water, ammonia, acetic acid) from the protonated molecule.
The new Autospec magnetic sector mass spectrometer is also equipped with a gas chromatograph and an autosampler allowing gas chromatographic separation of reaction or extraction mixtures prior to mass spectrometric analysis using either CI or EI as the ionization technique.
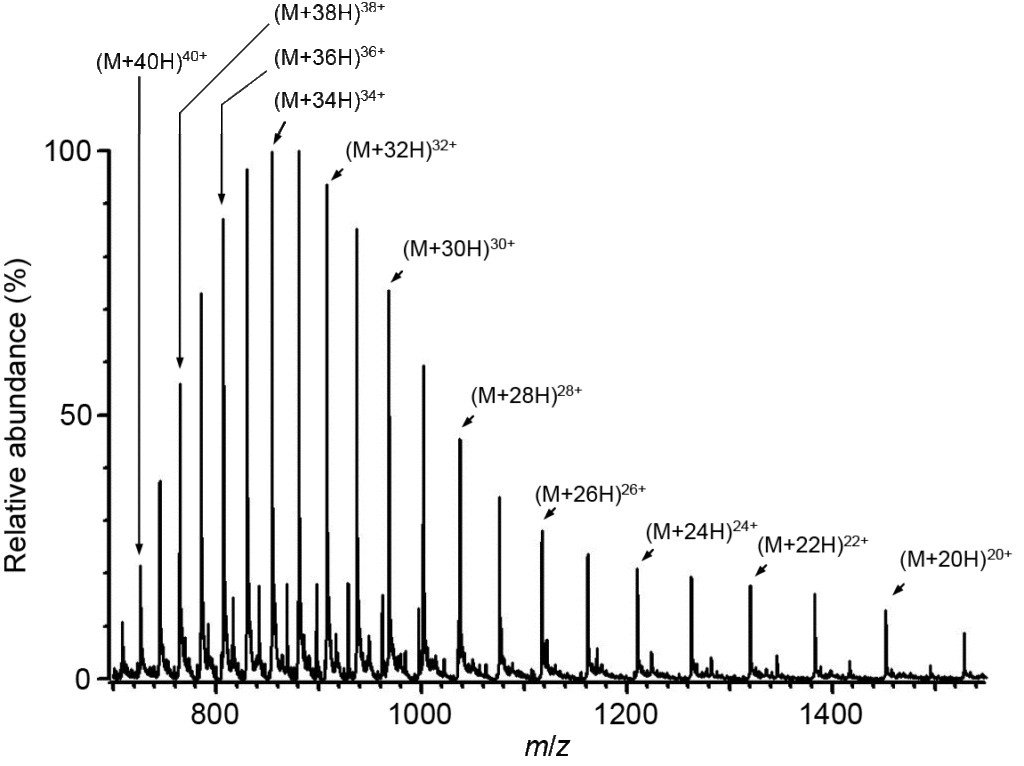
Electrospray ionization (ESI) generates ions from solution (typically an aqueous or aqueous/organic solvent system) by forming a fine spray of highly charged droplets in the presence of an electric potential (typically 1.0 to 3.5 kV). As a charged droplet decreases in size due to solvent evaporation, the electric charge density on its surface increases. The mutual repulsion between like charges on the surface eventually becomes so large that it exceeds the surface tension, causing the ejection of highly charged nanodroplets and/or solvated ions through what is known as a “Taylor cone”. Evaporation of the remaining solvent molecules results in unsolvated, gas-phase ions that are guided through ion optics into the mass analyzer. ESI can produce singly or multiply charged ions. The number of charges retained by a particular analyte depends on several factors such as the size, chemical composition, and higher order structure of the analyte molecule, the solvent composition, the presence of co-solutes, and the instrument parameters. For small molecules (< 2000 Da) ESI typically generates singly, doubly, or triply charged ions, while for large molecules (> 2000 Da) ESI can produce a series of multiply charged ions. Because mass spectrometers measure the mass-to-charge (m/z) ratio, the ESI mass spectrum for a large molecule typically contains multiple peaks corresponding to the different charge states. For example, the ESI mass spectrum of intact carbonic anhydase II (29,024 Da), shown below, exhibits charge states (M+19H)19+ through (M+41H)41+.
ESI is very suitable for a wide range of biochemical compounds including peptides and proteins, lipids, metabolites, oligosaccharides, and oligonucleotides. Bio-inorganic compounds, synthetic polymers, and intact non-covalent complexes can also be analyzed by ESI. ESI is extremely sensitive to impurities, detergents, salts, and buffers. Specific guidelines for preparing samples for ESI mass spectrometry can be obtained here. For bio-inorganic compounds the best results are usually obtained for samples that have been re-crystallized. Typical ESI solvents are methanol (MeOH), acetonitrile (AcN), water, and MeOH/water and AcN/water mixtures, although other solvents such as dimethylformamide (DMF), trichloromethane (CHCl3) and tetrahydrofuran (THF) can also be used if mixed with either MeOH or AcN. Please consult the Facility staff if you wish to submit a sample in a non-typical solvent.
The Synapt High Definition Ion Mobility Mass Spectrometer has an ion mobility analyzer which separates ions by collision cross section. Unlike conventional mass spectrometers, the ion mobility analyzer can separate ions that overlap in the mass-to-charge ratio (m/z) dimension. The ability to orthogonally separate ions that overlap in the m/z dimension translates into a larger analytical peak capacity to provide more molecular identifications for complex samples, e.g., mixtures of large numbers of peptides or proteins.

In addition, ion mobility spectrometry can reveal the presence of different conformers of a particular ion, which would be invisible to mass measurements alone. Understanding the higher order structure of biomolecules is important because different conformers may exhibit different biological activities.
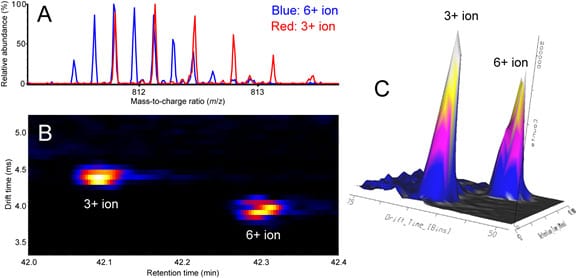
Ion mobility spectrometry separates ions that overlap in the mass-to-charge ratio dimension. (A) Overlay of mass spectra showing detail for overlapping isotopic distributions of the 3+ ion of a peptide (red) and 6+ ion of disulfide-linked peptide dimer (blue). (B) Two-dimensional heat map (ion mobility drift time vs. chromatographic retention time) showing resolved drift time distributions for the 3+ and 6+ ions. (C) Three-dimensional representation of drift time distributions of the 3+ and 6+ ions.
Training of New Users
As of March 7 2019, new users must be trained by Facility staff. Access to the MALDI
room (B208B Stanley Hall) will not be granted until the new user has completed a
training session with a staff member.New users interested in using the MALDI should contact Dr. Ulla Andersen by email:
norklit AT berkeley DOT edu
The MALDI instrument is a Voyager DE Pro from Applied Biosystems. It operates both in linear and reflector mode.
New sample plates can be obtained from JBI Scientific. The most common plates is a stainless steel plate with room for 100 samples. The catalogue number is V700666, the list price should be around $250.
In matrix-assisted laser desorption ionization (MALDI), the sample is embedded in a low molecular weight, UV-absorbing matrix that enhances desorption and ionization of the analyte. Sample preparation is crucial for successful MALDI. It is important to choose the correct matrix for your sample and to use suitable concentrations of both the sample and the matrix.
Commonly used MALDI matrices:
a-cyano-4-hydroxy-cinnamic acid (CHCA)
peptides, small proteins (<10 kDa)
protein digests
Sinapinic acid
Proteins (>10 kDa)
2,5-dihydroxy benzoic acid (DHB)
peptides, small proteins, small molecules, neutral carbohydrates, synthetic polymers, oligonucleotides (<10 bases)
2,4,6 trihydroxy acetophenone (THAP)
small oligonucleotides, acid sensitive compounds
Tips on how to spot your sample can be found here: MALDI Spotting Techniques_8-13-18.pptx
Tips on how to remove buffers, salts, urea, guanidine, EDTA, glycerol, DMSO and detergents from your MALDI spots can be found here: MALDI Clean-up_8-13-18.pptx
Groups that are interested in using the MALDI instrument should appoint a group operator. Each group is responsible for supplying their own sample plates, matrices and other supplies necessary for sample preparation (micropipettes, micro centrifuge tubes and solvents).
Alternatively you can contact the Facility Manager, Dr. Ulla N. Andersen and arrange to have a facility staff member analyze your MALDI samples.

