University of California, Berkeley, chemists have devised a novel method to selectively tag tryptophan residues within proteins, potentially leading to the development of new types of drugs and engineered proteins, including those that mediate protein-protein interactions.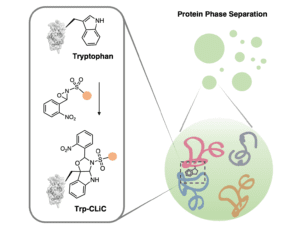
Led by Christopher J. Chang and F. Dean Toste, the Berkeley team drew inspiration from nature’s synthesis of indole alkaloids, devising an innovative N-sulfonyl oxaziridine reagent. This reagent reacts swiftly and selectively with tryptophan residues, offering a versatile tool for direct protein modification across proteomes without the need for light, electricity, or metal catalysts.
Read the full article of findings published by Chemical and Engineering News. Read this story on the Berkeley Chemistry website.