Graduate student Antonio Del Rio Flores studies Mycobacterium tuberculosis (Mtb) pathogenesis to explore the mechanism and inhibition of the isonitrile-forming enzyme Rv0097 that generates a virulence-related product.
Wenjun Zhang, a professor of Chemical and Biomolecular Engineering and a Chan Zuckerberg Biohub Investigator, currently leads a collaborative effort between UC Berkeley, MIT, and New York State Department of Health researchers that aims to study the mechanism of isonitrile formation by Rv0097 in order to design small-molecule inhibitors as potential anti-tuberculosis (TB) treatments.

Researchers from Zhang’s laboratory and their collaborators published several recent studies dealing with the identification of isonitrile-containing compounds in Mtb (including from Actinobacteria) and discovered a novel mechanism to isonitrile formation. The gene encoding the isonitrile-forming enzyme, Rv0097, has been extensively studied and linked to promote the formation of a virulence factor in Mtb from mutagenesis studies on mice.
However, an in-depth understanding of the mechanism associated with isonitrile formation and its implication to virulence remains unknown. A thorough understanding regarding the role of isonitriles towards TB pathogenicity may aid in the formulation of next generation anti-TB treatments that target isonitrile formation.
What does Mtb do and how can we combat it?
Mtb is the leading causative agent of TB, a disease that affects nearly ¼ of the world population resulting in over a million deaths per year. TB infections are prevalently present in lungs and many of these infections often lead to no symptoms as the bacteria is present in a latent state. However, active TB infections are associated with chronic cough and excessive weight loss. Mtb is a pathogen that relies on human-human transmission for its survival through infectious aerosol droplets originating from a cough, saliva, or a sneeze resembling the mode of transmission of COVID-19. TB has been a treatable disease for over half a century, yet scientists around the world have been unsuccessful at eradicating the disease. Conventional anti-TB drugs target biosynthetic processes of Mtb, the bacteria that causes this deadly disease. For example, rifampicin and isoniazid target RNA transcription and cell wall formation, respectively as their mode of action to eliminate bacteria. Treatment of this disease consists of a multi-drug regimen spanning several months to eliminate persistent bacteria in the lungs. Patient non-compliance to this strict regimen and emergent multi-drug resistant bacteria have motivated the scientific community in developing new anti-TB drug targets for the development of novel TB drug treatments.
How isonitriles can help inform our understanding of Mtb infection
The isonitrile functional group is present in over two hundred characterized natural products originating from marine and terrestrial organisms, which contain broad properties related to virulence, detoxification, metal acquisition, and antimicrobial activity. Isonitrile-bearing natural products have been isolated and characterized from diverse organisms such as fungi, bacteria, cyanobacteria, plants, marine sponges, and nudibranch mollusks. Notable isonitrile-containing natural products are the anti-viral agent xanthocillin from Penicillum notatum, the phenoloxidase inhibitor rhabduscin from Xenorhabdus, the isonitrile lipopeptide, SF2768, from Streptomyces thioluteus that mediates copper acquisition, and the compound amycomicin that exhibits prominent activity against Gram-Positive bacteria. Isonitriles possess the ability to coordinate transition metals due to their behavior as a carbon monoxide analog. Isonitriles also play an important role as building blocks in chemical synthesis for the development of multicomponent reactions.
“We recently revealed a new mechanism for isonitrile formation in nature,” Zhang says. “A non‐heme iron(II) and α-KG-dependent enzyme, Rv0097, was shown to catalyze the conversion of a single fatty acid substrate with a glycine adduct through oxidative decarboxylation.”

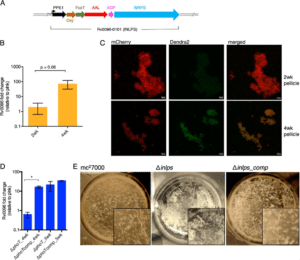
Previously, the only other known pathway to making isonitriles was by an enzyme family (IsnA) that condenses an amino acid and sugar to form the isonitrile group. Rv0097 is quite distinct from the IsnA family since the isonitrile group is formed on a single substrate utilizing an enzyme that utilizes iron(II) to perform this intriguing chemistry. Rv0097 is predicted to be encoded in an isonitrile lipopeptide (INLP) biosynthetic gene cluster (BGC) in Mtb with similar gene clusters present in pathogenic mycobacteria such as M. marinum and M. fortuitum, suggesting the virulence-associated nature of the product in Mycobacteria. Using both E. coli-based heterologous expression of homologous BGCs and biochemical analysis of encoded enzymes, the function of the BGC was revealed to produce an isonitrile lipopeptide. The biosynthesis has several unique features, including an unprecedented mechanism for isonitrile formation. However, the INLP metabolites obtained from heterologous expression were not identified from culture of Mtb and are presumably not native, bioactive metabolites. In addition, transcriptomic analysis revealed that the INLP BGC was minimally expressed in most common media, consistent with prior observations that this BGC was required for in vivo fitness of Mtb but not required for in vitro survival.
An important milestone in this project as noted by Zhang is to determine the chemical structures of mycobacterial INLPs. “Using a biofilm growth condition for Mtb and comparative metabolomics, we have detected endogenous INLP, which has a distinct mass compared to those of known INLPs,” Zhang says. This implies that the INLP native to Mtb has a novel structure with unknown modifications, thus motivating experiments to determine the structure of the native INLP and what machinery Mtb uses to make this complex molecule. Additionally, Zhang aims to define the role of INLP in pathogenesis and drug tolerance of Mtb in hosts. Using wild type, INLP deletion mutants, and mutants chemically complemented by INLP, the goal is to determine the role of INLP during acute and chronic phases of Mtb infection using a mouse model of infection.
Design and Testing of Small-Molecule Inhibitors for Rv0097
“We are interested in designing inhibitors for Rv0097 for multiple reasons,” Zhang says. “The encoding operon has been implicated in Mtb survival during both acute as well as chronic phases of infection, thus an inhibitor will facilitate a better understanding of Mtb pathogenicity and potentially lead to a treatment.
Additionally, Rv0097 was not required for in vitro survival of Mtb, suggesting that the pathogen may have less of a chance of developing drug resistances to inhibitors of this particular target and Rv0097 is distinct from any oxygenases in mammalian cells, thus a small-molecule inhibitor will have a lower risk of unspecific binding to other proteins and a higher success rate for further in vivo tests.”
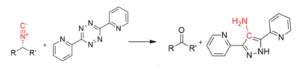
Based on the proposed catalytic mechanism and requirement of decarboxylation, we plan to synthesize and test small molecules with modified functional groups. The plan is to conduct an initial qualitative assessment consisting of a high-throughput colorimetric assay with tetrazine to monitor the production of isonitrile in real time. The candidate(s) showing a potent inhibitory activity will be selected for further measurements to assess their potential inhibitory capabilities.
“We hypothesize that these inhibitors will not influence Mtb planktonic growth, however, our recent collaboration with the Anil Ojha Lab revealed that INLP was produced in Mtb under biofilm growth and was required for Mtb biofilm maturation,” Zhang notes. “Under these biofilm conditions, Mtb possesses the ability to withstand 50 times the minimal inhibitory concentration of several antibiotics used to treat this disease!”
Zhang notes that based on preliminary findings, it is hypothesized that INLP-dependent growth of Mtb biofilms in host tissues leads to the development of persister cells, which are recalcitrant to current treatment regimens. Biofilms are three-dimensional, sessile, matrix-encapsulated, and multi-cellular communities that grow at liquid-air interfaces. Biofilm development involves a complex genetically programmed process in which bacteria communicate with each other to form these architectures based on specific environment cues, nutrient limitations, and in response to specific stressors such as antibiotics. The study of biofilms is especially relevant to human health since many biofilm-associated diseases exist such as cystic fibrosis, urinary tract infections, dental plaque, and catheter infections.
Given the role of INLPs in Mtb pathogenesis and biofilm formation, targeting its biosynthesis is a potential and promising target for inhibiting INLP-dependent persistence specifically through the action of Rv0097. The Zhang lab recently received an AIMS award from Atomwise, which utilized an artificial-intelligence (AI) based compound screening method to identify small-molecule inhibitors against Rv0097 based on recently acquired crystal structures obtained by the Drennan lab. Such inhibitors are expected to serve as biological probes to study the role of INLP as well as potentially new therapeutics against persistent Mtb.