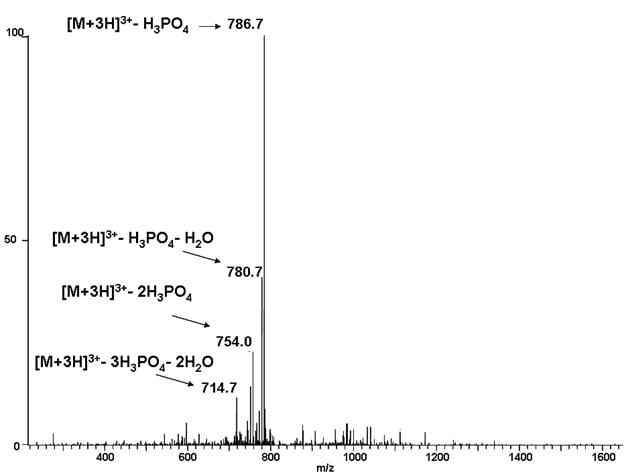
LC MS/MS data can be processed to detect any modifications that may be present in the peptides detected. The modification must add a calculatable molecular weight. Modification analysis can be performed using either one dimensional or multi dimensional “MudPIT” chromatography depending on the sample complexity. We can often find modifications on a target protein even when it is part of a complex mixture. Often modifications of interest can be found simply by searching LC mass spec data collected under standard conditions, but we can do better if the data collection is planned specifically with modification analysis in mind. For some types of modification including phosphorylation, we can collect additional data by CID, HCD or ETD fragmentation that confirms the presence of the modification with higher confidence. Ask us for details.
In some cases, chances of pinpointing the site of a modification increase if overlapping peptides are analyzed or if an enzyme other than trypsin is used to generate peptides. To produce sets of overlapping peptides, we recommend digestion with two or three proteases that vary in their specificity. If there is a suspected site of modification, the proteolytic digest can be planned to produce peptides of optimal mass containing that site. You can use peptidecutter to choose an appropriate protease.
To perform this type of analysis, please refer to the following forms and protocols:
Lys-C or trypsin in-solution protein digest protocol
From the J. Yates Lab, Scripps Research Institute
This protocol can be adapted to use other proteases. Change the buffer from Tris to one that can
give a pH appropriate for the desired protease, then follow the trypsin directions.
1. Bring solution up to 8M Urea and 100mM Tris-HCl pH 8.5 (use 10 M urea and 1 M tris
stocks; ideal final volume is about 80μl).
2. Add 100 mM TCEP (a reducing agent) to a final concentration of 5 mM. Incubate at room
temp. for 20 min.
3. Add 500mM iodoacetamide (make fresh daily) to a final concentration of 10 mM. Incubate
at room temp. for 15 min. in the dark (covered with foil).
4. Use one of the following enzymes:
Lys-C Digest:
1. Add in Lyse-C 1μl (0.1μg/μl), 1/100th total amount.
2. Incubate for 4 hr. at 37°C in the dark.
Trypsin Digest:
1. Dilute samples by a factor of four with 100mM Tris-HCl pH 8.5 (final urea conc. = 2M)
2. Add 100 mM CaCl2 to a final conc. of 1mM.
3. Add in trypsin 1μl (0.5μg/μl)
4. Incubate overnight at 37°C in the dark.
5. Add formic acid to 5% final concentration. Use only glass pipets for adding concentrated
formic acid.
Solutions:
1M TCEP
for 1ml:
287mg
1ml MilliQ water
make a 1/10 dilution and store at –20°C in aliquots
500mM iodoacetamide
for 0.5ml:
46mg
500μl ddH2O, make fresh
1M CaCl2
for 100ml:
14.7g CaCl2•2H2O
ddH2 O to 100ml
TCA precipitation of proteins
Protocol prepared by Lori Kohlstaedt.
1. Add 100% (w/v) TCA (trichloro acetic acid, see preparation method below) to the
sample to bring the TCA concentration to 20%.
2. Incubate on ice for at least 1 hr. Dilute samples may be left overnight.
3. Spin at maximum speed at 4 degrees in a microcentrifuge for 10 min.
4. Wash the pellet 3X with a solution of ice cold 0.01 M HCl / 90% acetone.
5. Allow the pellet to air dry.
The pellet can then be resuspended directly in 100 mM tris, pH 8.5, 8 M urea for
enzymatic digestion for mass spectrometry
Preparation of 100% TCA:
(Don’t try to weigh out TCA; it’s too hygroscopic)
1. Obtain a fresh bottle of crystalline TCA.
2. Read the weight in the container from the label.
3. Add distilled water to give a 100 g / 100 ml solution at final volume.
Store the solution in an acid compatible container.
Another common problem is the introduction into the sample of contaminants that leach out of plastics. Pay close attention to solvent compatibilities of tubes and pipets you use during sample preparation. For example, do not pipet concentrated acid with plastic pipet tips.
Sample desalting for mass spectrometry
Equipment:
C18 Spec tips, Agilent, cat #A57203
p200 and p1000 pipetmen
You can use a p1000 pipetman to wash and elute spec tips. For each wash, add the
required volume into the top of the spec tip, then press a p1000 tip firmly into the spec tip
and depress the plunger to drive the liquid through the device.
1. Wet the spec tip by pushing through 200 μl of HPLC grade methanol.
2. Wash the spec tip with 3 x 200μl 5% acetonitrile/5% formic acid.
3. If your sample does not already contain an acidic ion pairing agent, add formic
acid to your sample to 5%. NOTE: Do not pipet concentrated formic acid with
plastic pipet tips; use glass.
4. Push the sample through the spec tip. You may pass the sample through twice,
washing with 5% acetoniltrile/5% formic acid between each pass, if you would
like to maximize binding.
5. Wash with 3 x 200 μl 5% acetonitrile/ 5% formic acid.
6. Elute with 2 x 100 μl 80% acetonitrile/5% formic acid.
7. Speed vac the sample to dryness.
Reagents:
HPLC grade methanol
5% HPLC grade acetonitrile/5% formic acid
80% HPLC grade acetonitrile/5% formic acid
Make solutions with MilliQ or better water. NOTE: Do not pipet concentrated formic
acid with plastic pipet tips; use glass.
We urge you to contact facility staff to discuss your project prior to sample preparation.