Jump to section:
Key Concepts
Proteins need to fold correctly in order to be functional. In our case, GFP will only fluoresce when folded properly. Because proteins have evolved to function in specific environments, environmental conditions like oxygen concentration and pH can have a significant impact on protein folding and thus protein function. When proteins are challenged with different environmental conditions, they often denature (unfold). Each protein is unique, so special conditions may be required to prompt them to refold correctly.
During folding, GFP requires molecular oxygen (O2) to oxidize residues S65, Y66, and G67 to form its potent chromophore. Your research advisor wants you to use GFP to label an oral biologic that needs to be active in the intestine of mice. Unfortunately, you can’t engineer the epithelial cells of the mice to express GFP because there is not enough O2 present in the mouse intestine for the chromophore to develop properly.
To get around this, you need to deliver the GFP orally, but you are concerned that the mature GFP won’t survive the acidic pH of the stomach and still be able to fluoresce when it reaches the intestine. We will be testing the ability of GFP to withstand an acid challenge to find out if GFP can refold and fluoresce after being exposed to acid.
Materials
- 150μL purified GFP protein
- 100mM citric acid
- 200mM sodium phosphate dibasic solution
- Tris Buffer (250 mM Tris, pH 8.0)
Procedure
- Check that you have at least 150µL of your GFP elution remaining. If not, dilute with Wash buffer (from protein purification) to 150µL.
- Use your Wash buffer for the buffer at pH 8. Use the following table to create 100µL of buffers with pH 6, pH 4, pH 2, and pH 1:
|
pH |
µL 0.1 M citric acid |
µL 0.2 M sodium phosphate |
|
1 |
100 |
0 |
|
2 |
90 |
10 |
|
4 |
55 |
45 |
|
6 |
25 |
75 |
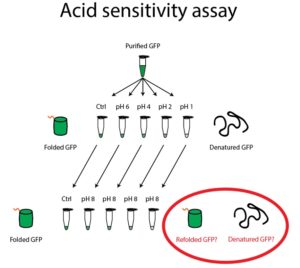
- Label five clear Eppendorf tubes “GFP, pH 8”, “GFP, pH 6”, “GFP, pH 4”, “GFP, pH 2”, and “GFP, pH 1”. Aliquot 25μL of GFP into these five tubes.
- Add 25μL of Wash Buffer pH 8 to the GFP in the tube labeled pH 8.
- Add 75μL of the appropriate citric acid/sodium phosphate buffer to the GFP in the four acidic tubes. Incubate these tubes for 2 minutes.
- At the end of the incubation, check these five tubes for GFP fluorescence by using the Safe Imager to detect the presence or absence of GFP fluorescence.
- Label five Eppendorf tubes “8 🡪 8”, “6 🡪 8”, “4 🡪 8”, “2 🡪 8”, and “1 🡪 8”. Add 50μL of the Tris Buffer to each of the five tubes.
- Add 25μL of your acidic GFP solutions from step 2 into these five tubes. Incubate these tubes for 5 minutes.
- At the end of the incubation, check all ten tubes for GFP fluorescence by using the Safe Imager to detect the presence or absence of GFP fluorescence in each tube. Do the tubes that have been exposed to acid fluoresce at the same intensity as the pH 8 tube? Why do you think this is the case?
- Optional: Add 80μL of Wash Buffer to the following 12 wells of your BCA microtiter plate: G1-6, and H1-6. Add 20μL of your GFP solutions or wash buffer to the wells in this order:
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
… |
|
|
G |
Wash | GFP pH 1 | GFP pH 2 | GFP pH 4 | GFP pH 6 | GFP pH 8 | – | – | – |
|
H |
Wash | 1 🡪 8 | 2 🡪 8 | 4 🡪 8 | 6 🡪 8 | 8 🡪 8 | – | – | – |
Measure the fluorescence of each well with the excitation at 488nm and the emission at 510nm. Create a stacked bar graph with pH along the x axis and fluorescence in RFU along the y axis.
What are the conclusions that you should report to your research advisor? Is it feasible to attach GFP to a biologic that must pass through the stomach? What conditions need to be investigated further to improve on this preliminary result that you just obtained?